Research
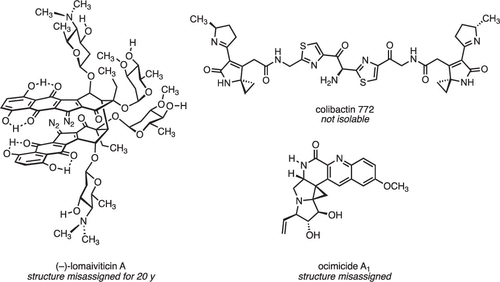
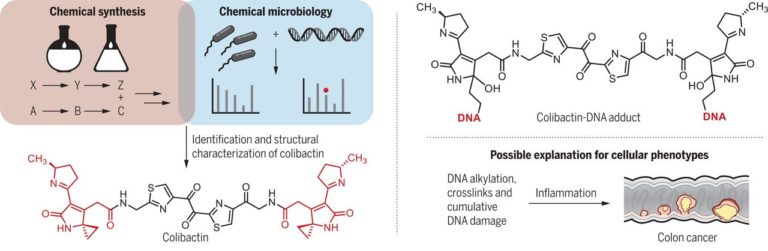
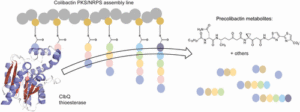
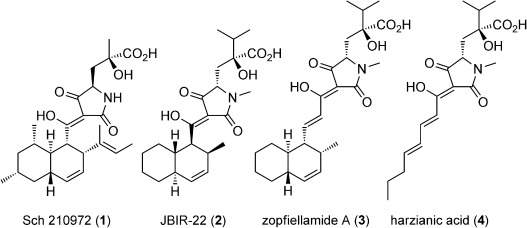
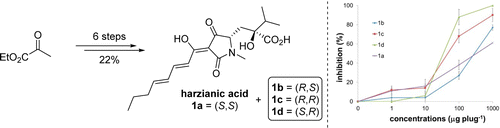
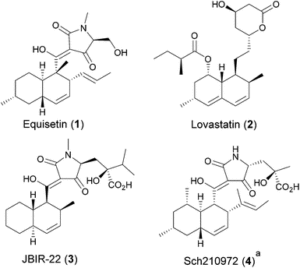
The complex architecture and exquisite bioactivity of natural products has long inspired and invigorated synthetic chemists. The field has evolved to the point where arguably with the necessary resources and time the synthesis of any natural product is achievable. The wealth of natural product reserves still to be studied and the constant need for new therapeutic agents has motivated chemists to develop innovative methods such as iterative synthesis, diversity orientated synthesis and automation to accelerate and streamline natural product synthesis. Nature provides us with the most efficient strategy of all, producing infinite collections of diverse biopolymers such as peptides, DNA, oligosaccharides and natural products using an iterative assembly line approach. Inspired by this, the Healy lab is developing an automated assembly line synthesis of polyketides and non-ribosomal peptide natural products. We aim to combine strategies inspired by natural product biosynthesis with the tools and techniques developed for the automated synthesis of other biopolymers to tackle the complex task of automating natural product synthesis. Together with collaborators, the goal is to develop a streamlined end-to-end process from genome sequencing to structure prediction, synthesis, bioactivity screening and preclinical development.


Publications
22. Thiocarboxylic S-Acid, Selenocarboxylic Se-Acid, and Tellurocarboxylic Te-Acid Derivatives (Update 2024)
Cellnik, T., Jo, W., Healy, A., Science of Synthesis Knowledge Updates, early view. DOI: 10.1055/sos-SD-120-00188
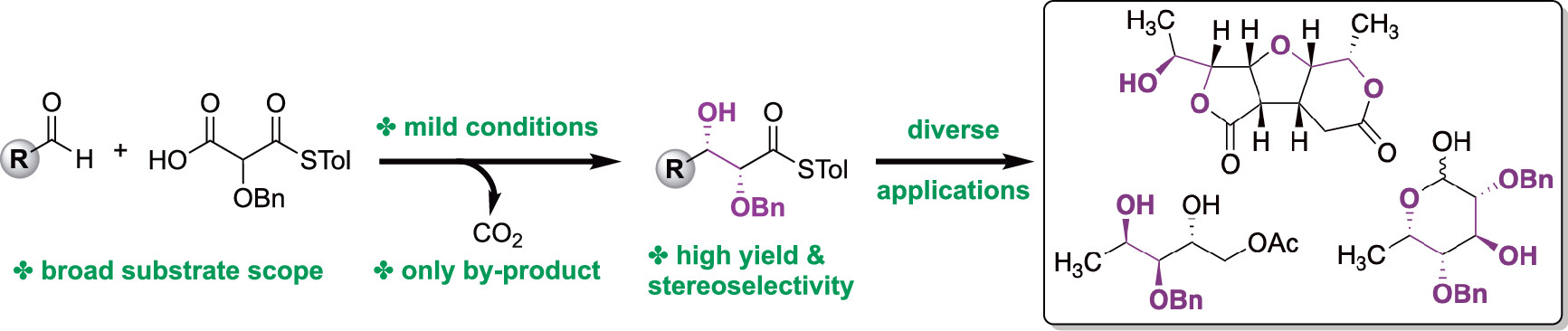
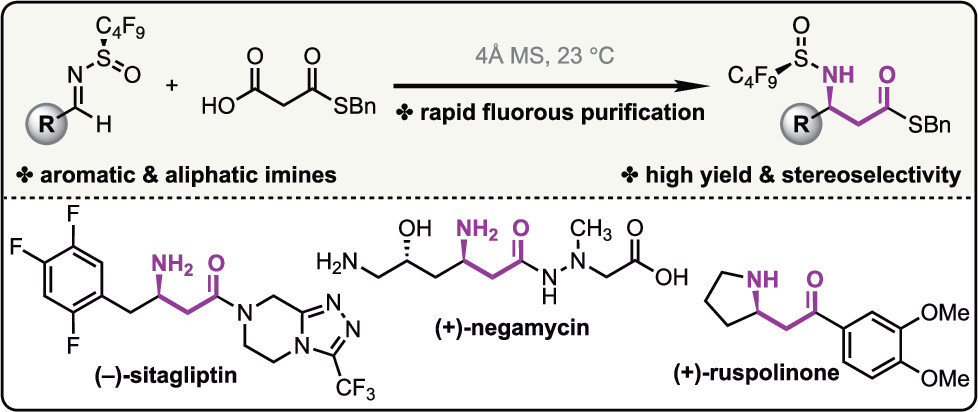
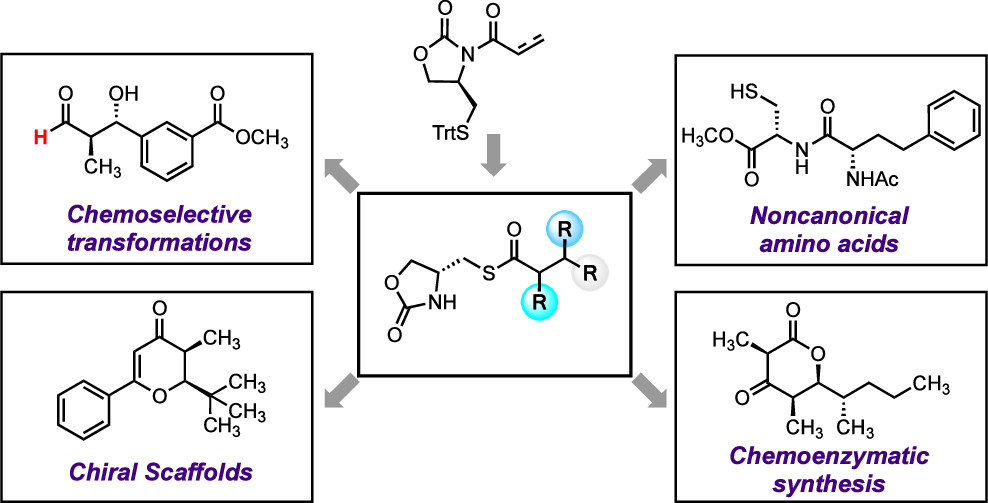
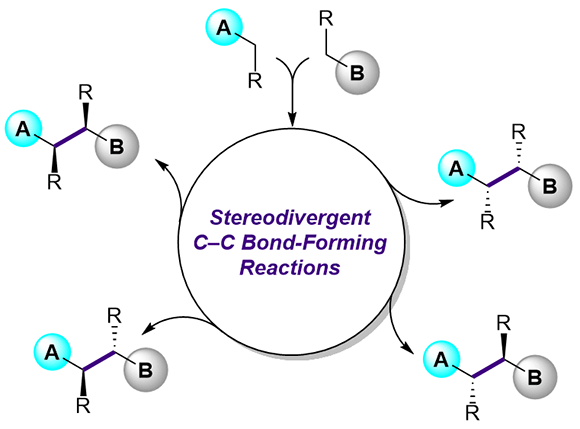
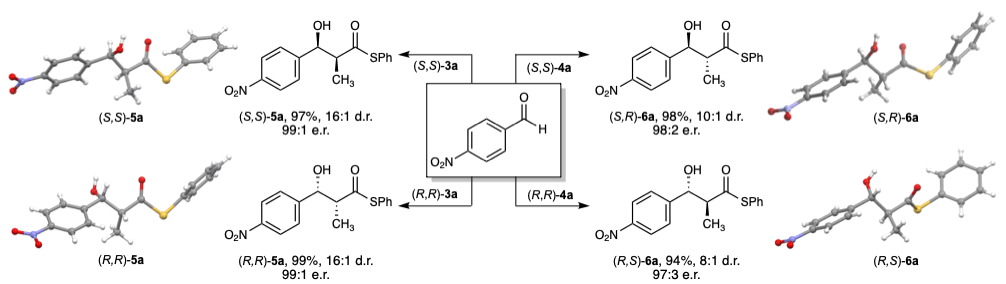
19. A catalytic enantioselective stereodivergent aldol reaction
Md. Ataur Rahman, Torsten Cellnik, Brij Bhushan Ahuja, Liang Li, Alan R. Healy*, Sci. Adv., 2023, 9, eadg8776.
highlighted in: “Powerful approach to C–C bond formation”, Science, 2023, 379, 6637, 1110.
highlighted in: Synform, 2023/07, A125–A127.
highlighted in: Synfacts, 2023, 19(07), 0690.
15. An inter-subunit protein-peptide interface that stabilizes the specific activity and oligomerization of the AAA+ chaperone Reptin
Dominika Coufalova, Lucy Remnant, Lenka Hernychova, Petr Muller, Alan Healy, Srinivasaraghavan Kannan, Nicholas Westwood, Chandra S. Verma, Borek Vojtesek, Ted R. Huppa*, Douglas R. Houston, Journal of Proteomics, 2019, 199, 89–101.
13. A DHODH inhibitor increases p53 synthesis and enhances tumor cell killing by p53 degradation blockage
Marcus J. G. W. Ladds, Ingeborg M. M. van Leeuwen, Catherine J. Drummond, Su Chu, Alan R. Healy, Gergana Popova, Andrés Pastor Fernández, Tanzina Mollick, Suhas Darekar, Saikiran K. Sedimbi, Marta Nekulova,Marijke C. C. Sachweh, Johanna Campbell, Maureen Higgins, Chloe Tuck, Mihaela Popa, Mireia Mayoral Safont, Pascal Gelebart, Zinayida Fandalyuk, Alastair M. Thompson, Richard Svensson, Anna-Lena Gustavsson, Lars Johansson, Katarina Färnegårdh, Ulrika Yngve, Aljona Saleh, Martin Haraldsson, Agathe C. A. D’Hollander, Marcela Franco, Yan Zhao, Maria Håkansson, Björn Walse, Karin Larsson, Emma M. Peat, Vicent Pelechano, John Lunec, Borivoj Vojtesek, Mar Carmena, William C. Earnshaw, Anna R. McCarthy, Nicholas J. Westwood, Marie Arsenian-Henriksson, David P. Lane, Ravi Bhatia, Emmet McCormack, and Sonia Laín, Nature Communications, 2018, 9, 1107.
2. Discovery of a novel ligand that modulates the protein–protein interactions of the AAA+ superfamily oncoprotein reptin
Alan R. Healy, Douglas R. Houston *, Lucy Remnant, Anne-Sophie Huart, Veronika Brychtova, Magda M. Maslon, Olivia Meers, Petr Muller, Adam Krejci, Elizabeth A. Blackburn, Borek Vojtesek, Lenka Hernychova, Malcolm D. Walkinshaw, Nicholas J. Westwood *, Ted R. Hupp *, Chem. Sci., 2015, 6, 3109-3116.
1. Synthesis and Structure−Activity Analysis of New Phosphonium Saltswith Potent Activity against African Trypanosomes
Andrea Taladriz,# Alan Healy,# Eddysson J. Flores Pérez,# Vanessa Herrero García, Carlos Ríos Martínez, Abdulsalam A. M. Alkhaldi,# Anthonius A. Eze, Marcel Kaiser, Harry P. de Koning, Antonio Chana, Christophe Dardonville *, J. Med. Chem., 2012, 55, 2606−2622.
Patents
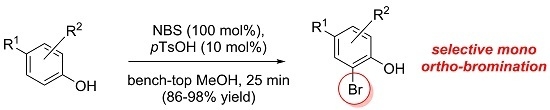
The present invention is directed to novel anticancer compounds which show excellent activity as anticancer agents. The present invention is also directed to pharmaceutical compositions based upon these compounds and methods of treating cancer, including drug resistant, multiple drug resistant, metastatic and recurrent cancer.
Seth Herzon, Alan Healy, US10316037B1.
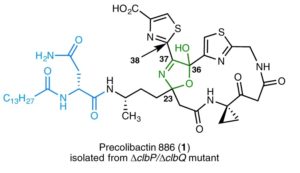
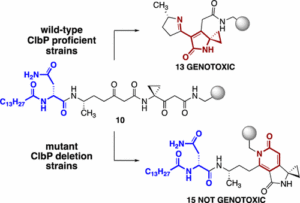
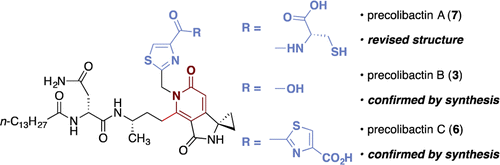
The present invention is directed to compounds related to precolibactin, pharmaceutical compositions based upon these compounds and methods of synthesis which are employed to provide intermediates and final compounds, which are principally alkylating agents and anticancer compounds. The chemical synthetic approach disclosed facilitates the synthesis of numerous precolibactin analogs which can be used in the treatment of cancer.
Seth Herzon, Alan Healy, Jason Crawford, Maria Vizcaino, Herman Nikolayevskiy, WO2017132459A1.